
Valproate (VPA), and its valproic acid, sodium valproate, and valproate semisodium forms, are medications primarily used to treat epilepsy and bipolar disorder and to prevent migraine headaches. It is useful for the prevention of seizures in those with absence seizures, partial seizures, and generalized seizures. It can be given intravenously or by mouth. Long and short acting formulation of tablets exist.
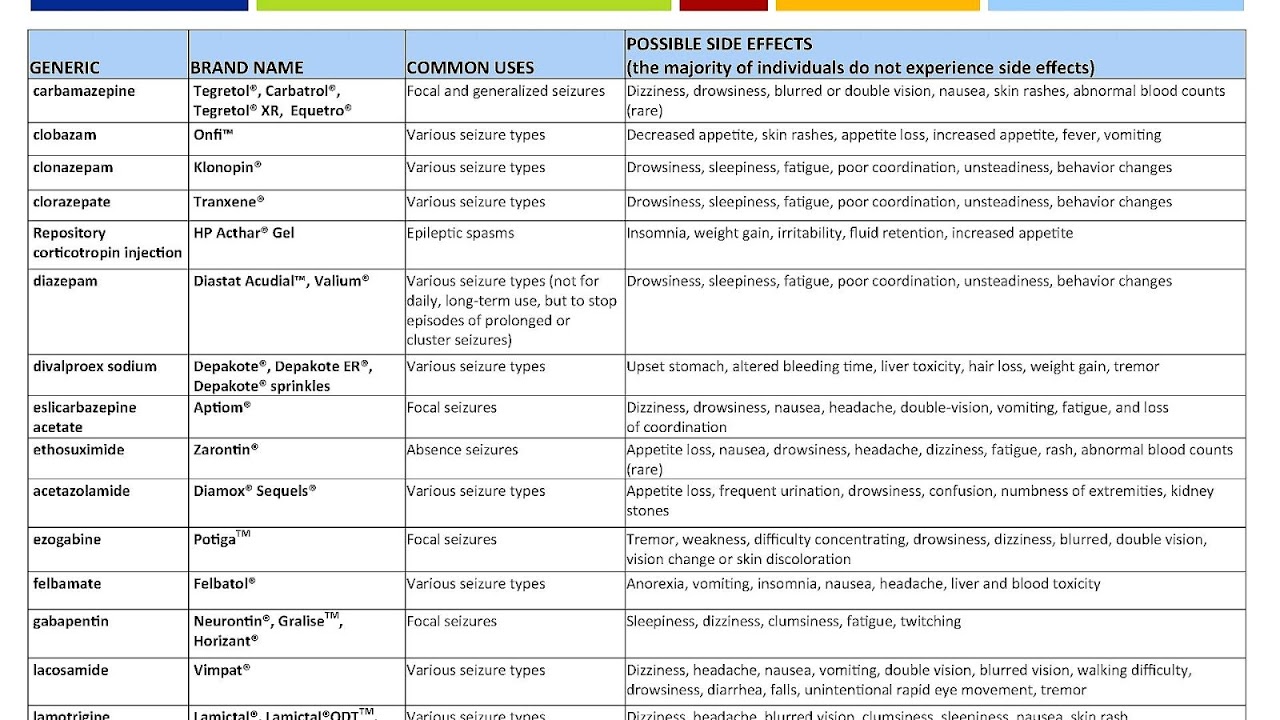
Common side effects include nausea, vomiting, sleepiness, and a dry mouth. Serious side effects can include liver problems and regular monitoring of liver function tests is therefore recommended. Other serious risks include pancreatitis and an increased suicide risk. It is known to cause serious abnormalities in the baby if taken during pregnancy. Because of this it is not typically recommended in women of childbearing age who have migraines.
It is unclear exactly how valproate works. Proposed mechanisms include affecting GABA levels, blocking voltage-gated sodium channels, and inhibiting histone deacetylases. Valproic acid is a branched short-chain fatty acid (SCFA) made from valeric acid.
Valproate was first made in 1881 and it came into medical use in 1962. Valproate is included in the World Health Organization's List of Essential Medicines, the most effective and safe medicines needed in a health system. It is available as a generic medication. The wholesale cost in the developing world is between 0.14 and 0.52 USD per day. In the United States, it costs roughly $0.90 USD per day. It is marketed under the brand names Depakote and Epilim among others.

Maps, Directions, and Place Reviews
Terminology
Valproic acid (VPA) is an organic weak acid. The conjugate base is valproate. The sodium salt of the acid is sodium valproate and a coordination complex of the two is known as divalproex sodium.
Depakote Side Effects Video
Medical uses
It is used primarily to treat epilepsy and bipolar disorder. It is also used to prevent migraine headaches.
Epilepsy
Valproate has a broad spectrum of anticonvulsant activity, although it is primarily used as a first-line treatment for tonic-clonic seizures, absence seizures and myoclonic seizures and as a second-line treatment for partial seizures and infantile spasms. It has also been successfully given intravenously to treat status epilepticus.
Mental illness
Bipolar disorder
Valproate products are also used to treat manic or mixed episodes of bipolar disorder.
Schizophrenia
A 2016 systematic review compared the efficacy of valproate as an add-on for people with schizophrenia:
Dopamine dysregulation syndrome
Valproic acid may have efficacy in controlling the symptoms of the dopamine dysregulation syndrome that arise from the treatment of Parkinson's disease with dopamine receptor agonists.
Migraines
Valproate is also used to prevent migraine headaches. Because this medication can be potentially harmful to the fetus, valproate should be considered for women of childbearing potential only after the risks have been discussed.
Other
The medication has been tested in the treatment of AIDS and cancer, owing to its histone deacetylase-inhibiting effects.

Adverse effects
Valproic acid has a black box warning for hepatotoxicity, pancreatitis, and fetal abnormalities.
Other possible side effects
There is evidence that valproic acid may cause premature growth plate ossification in children and adolescents, resulting in decreased height. Valproic acid can also cause mydriasis, a dilation of the pupils. There is evidence that shows valproic acid may increase the chance of polycystic ovary syndrome (PCOS) in women with epilepsy or bipolar disorder. Studies have shown this risk of PCOS is higher in women with epilepsy compared to those with bipolar disorder.
Pregnancy
Valproate causes birth defects; exposure during pregnancy is associated with about three times as many major abnormalities as usual, mainly spina bifida with the risks being related to the strength of medication used and use of more than one drug. More rarely, with several other defects, possibly including a "valproate syndrome". Characteristics of this valproate syndrome include facial features that tend to evolve with age, including a triangle-shaped forehead, tall forehead with bifrontal narrowing, epicanthic folds, medial deficiency of eyebrows, flat nasal bridge, broad nasal root, anteverted nares, shallow philtrum, long upper lip and thin vermillion borders, thick lower lip and small downturned mouth. While developmental delay is usually associated with altered physical characteristics (dysmorphic features), this is not always the case.
Children of mothers taking valproate during pregnancy are at risk for lower IQs. Maternal valproate use during pregnancy has been associated with a significantly higher probability of autism in the offspring. A 2005 study found rates of autism among children exposed to sodium valproate before birth in the cohort studied were 8.9%. The normal incidence for autism in the general population is estimated at less than one percent. A 2009 study found that the 3-year-old children of pregnant women taking valproate had an IQ nine points lower than that of a well-matched control group. However, further research in older children and adults is needed.
Sodium valproate has been associated with the rare condition paroxysmal tonic upgaze of childhood, also known as Ouvrier-Billson syndrome, from childhood or fetal exposure. This condition resolved after discontinuing valproate therapy.
Women who intend to become pregnant should switch to a different medication if possible, or decrease their dose of valproate. Women who become pregnant while taking valproate should be warned that it causes birth defects and cognitive impairment in the newborn, especially at high doses (although valproate is sometimes the only drug that can control seizures, and seizures in pregnancy could have even worse consequences.) Studies have shown that taking folic acid can reduce the risk of congenital neural tube defects.
Elderly
Valproate in elderly people with dementia caused increased sleepiness. More people stopped the medication for this reason. Additional side effects of weight loss and decreased food intake was also associated in one half of people who become sleepy.
Contraindications
Contraindications include:
- Pregnancy
- Pre-existing acute or chronic liver dysfunction or family history of severe liver inflammation (hepatitis), particularly medicine related.
- Known hypersensitivity to valproate or any of the ingredients used in the preparation
- Urea cycle disorders
- Hepatic porphyria
- Hepatotoxicity
- Mitochondrial disease
- Pancreatitis
- Porphyria
Interactions
Valproate inhibits CYP2C9, glucuronyl transferase, and epoxide hydrolase and is highly protein bound and hence may interact with drugs that are substrates for any of these enzymes or are highly protein bound themselves. It may also potentiate the CNS depressant effects of alcohol. It should not be given in conjunction with other antiepileptics due to the potential for reduced clearance of other antiepileptics (including carbamazepine, lamotrigine, phenytoin and phenobarbitone) and itself. It may also interact with:
- Aspirin: may increase valproate concentrations. May also interfere with valproate's metabolism.
- Benzodiazepines: may cause CNS depression and there are possible pharmacokinetic interactions.
- Carbapenem antibiotics: reduces valproate levels, potentially leading to seizures.
- Cimetidine: inhibits valproate's metabolism in the liver, leading to increased valproate concentrations.
- Erythromycin: inhibits valproate's metabolism in the liver, leading to increased valproate concentrations.
- Ethosuximide: may increase ethosuximide concentrations and lead to toxicity.
- Felbamate: may increase plasma concentrations of valproate.
- Mefloquine: may increase valproate metabolism combined with the direct epileptogenic effects of mefloquine.
- Oral contraceptives: may reduce plasma concentrations of valproate.
- Primidone: may accelerate metabolism of valproate, leading to a decline of serum levels and potential breakthrough seizure.
- Rifampin: increases the clearance of valproate, leading to decreased valproate concentrations
- Warfarin: may increase warfarin concentration and prolong bleeding time.
- Zidovudine: may increase zidovudine serum concentration and lead to toxicity.
Overdose and toxicity
Excessive amounts of valproic acid can result in sleepiness, tremor, stupor, respiratory depression, coma, metabolic acidosis, and death. In general, serum or plasma valproic acid concentrations are in a range of 20-100 mg/l during controlled therapy, but may reach 150-1500 mg/l following acute poisoning. Monitoring of the serum level is often accomplished using commercial immunoassay techniques, although some laboratories employ gas or liquid chromatography. In contrast to other antiepileptic drugs, at present there is little favorable evidence for salivary therapeutic drug monitoring. Salivary levels of valproic acid correlate poorly with serum levels, partly due to valproate's weak acid property (pKa of 4.9).
In severe intoxication, hemoperfusion or hemofiltration can be an effective means of hastening elimination of the drug from the body. Supportive therapy should be given to all patients experiencing an overdose and urine output should be monitored. Supplemental L-carnitine is indicated in patients having an acute overdose and also prophylactically in high risk patients. Acetyl-L-carnitine lowers hyperammonemia less markedly than L-carnitine.

Pharmacology
Pharmacodynamics
Although the mechanism of action of valproate is not fully understood, traditionally, its anticonvulsant effect has been attributed to the blockade of voltage-gated sodium channels and increased brain levels of gamma-aminobutyric acid (GABA). The GABAergic effect is also believed to contribute towards the anti-manic properties of valproate. In animals, sodium valproate raises cerebral and cerebellar levels of the inhibitory synaptic neurotransmitter, GABA, possibly by inhibiting GABA degradative enzymes, such as GABA transaminase, succinate-semialdehyde dehydrogenase and by inhibiting the re-uptake of GABA by neuronal cells.
Prevention of neurotransmitter-induced hyperexcitability of nerve cells, via Kv7.2 channel and AKAP5, may also contribute to its mechanism. Also, it has been shown to protect against a seizure-induced reduction in phosphatidylinositol (3,4,5)-trisphosphate (PIP3) as a potential therapeutic mechanism.
It also has histone deacetylase-inhibiting effects. The inhibition of histone deacetylase, by promoting more transcriptionally active chromatin structures, likely presents the epigenetic mechanism for regulation of many of the neuroprotective effects attributed to valproic acid. Intermediate molecules mediating these effects include VEGF, BDNF, and GDNF.
Endocrine actions
Valproic acid has been found to be an antagonist of the androgen and progesterone receptors, and hence as a nonsteroidal antiandrogen and antiprogestogen, at concentrations much lower than therapeutic serum levels. In addition, the drug has been identified as a potent aromatase inhibitor, and suppresses estrogen concentrations. These actions are likely to be involved in the reproductive endocrine disturbances seen with valproic acid treatment.
Valproic acid has been found to directly stimulate androgen biosynthesis in the gonads via inhibition of histone deacetylases and has been associated with hyperandrogenism in women and increased 4-androstenedione levels in men. High rates of polycystic ovary syndrome and menstrual disorders have also been observed in women treated with valproic acid.
Pharmacokinetics
The vast majority of valproate metabolism occurs in the liver. In adult patients taking valproate alone, 30-50% of an administered dose is excreted in urine as a glucuronide conjugate. The other major pathway in the metabolism of valproate is mitochondrial beta-oxidation, which typically accounts for over 40% of an administered dose. Typically, less than 20% of an administered dose is eliminated by other oxidative mechanisms. Less than 3% of an administered dose of valproate is excreted unchanged (i.e., as valproate) in urine.

Chemistry
Valproic acid is a branched short-chain fatty acid and a derivative of valeric acid.

History
Valproic acid was first synthesized in 1882 by Beverly S. Burton as an analogue of valeric acid, found naturally in valerian. Valproic acid is a carboxylic acid, a clear liquid at room temperature. For many decades, its only use was in laboratories as a "metabolically inert" solvent for organic compounds. In 1962, the French researcher Pierre Eymard serendipitously discovered the anticonvulsant properties of valproic acid while using it as a vehicle for a number of other compounds that were being screened for antiseizure activity. He found it prevented pentylenetetrazol-induced convulsions in laboratory rats. It was approved as an antiepileptic drug in 1967 in France and has become the most widely prescribed antiepileptic drug worldwide. Valproic acid has also been used for migraine prophylaxis and bipolar disorder.

Society and culture
Cost
It is available as a generic medication. The wholesale cost in the developing world is between $0.14 and $0.52 USD per day. In the European Union, end-user costs are less than 0.60 EUR for an average daily dose in Germany. In the United States, it costs about $0.90 USD per day.
Approval status
Formulations
Valproate exists in two main molecular variants: sodium valproate and valproic acid without sodium (often implied by simply valproate). A mixture between these two is termed semisodium valproate. It is unclear whether there is any difference in efficacy between these variants, except from the fact that about 10% more of sodium valproate is needed than valproic acid without sodium to compensate for the sodium itself.
Brand names of valproic acid
Branded products include:
Brand names of sodium valproate
Portugal
- Tablets - Diplexil-R by Bial.
United States
- Intravenous injection - Depacon by Abbott Laboratories.
- Syrup - Depakene by Abbott Laboratories. (Note Depakene capsules are valproic acid).
- Depakote tablets are a mixture of sodium valproate and valproic acid.
- Tablets - Eliaxim by Bial.
Australia
- Epilim Crushable Tablets Sanofi
- Epilim Sugar Free Liquid Sanofi
- Epilim Syrup Sanofi
- Epilim Tablets Sanofi
- Sodium Valproate Sandoz Tablets Sanofi
- Valpro Tablets Alphapharm
- Valproate Winthrop Tablets Sanofi
- Valprease tablets Sigma
New Zealand
- Epilim by Sanofi-Aventis
All the above formulations are Pharmac-subsidised.
UK
- Depakote Tablets (as in USA)
- Tablets - Orlept by Wockhardt and Epilim by Sanofi
- Oral solution - Orlept Sugar Free by Wockhardt and Epilim by Sanofi
- Syrup - Epilim by Sanofi-Aventis
- Intravenous injection - Epilim Intravenous by Sanofi
- Extended release tablets - Epilim Chrono by Sanofi is a combination of sodium valproate and valproic acid in a 2.3:1 ratio.
- Enteric-coated tablets - Epilim EC200 by Sanofi is a 200-mg sodium valproate enteric-coated tablet.
UK only
- Capsules - Episenta prolonged release by Beacon
- Sachets - Episenta prolonged release by Beacon
- Intravenous solution for injection - Episenta solution for injection by Beacon
Germany, Switzerland, Norway, Finland, Sweden
- Tablets - Orfiril by Desitin Pharmaceuticals
- Intravenous injection - Orfiril IV by Desitin Pharmaceuticals
South Africa
- Syrup - Convulex by Byk Madaus
- Tablets - Epilim by Sanofi-synthelabo
Malaysia
- Tablets - Epilim by Sanofi-Aventis
Romania
- Companies are SANOFI-AVENTIS FRANCE, GEROT PHARMAZEUTIKA GMBH and DESITIN ARZNEIMITTEL GMBH
- Types are Syrup, Extended release mini tablets, Gastric resistant coated tablets, Gastric resistant soft capsules, Extended release capsules, Extended release tablets and Extended release coated tablets
Canada
- Intravenous injection - Epival or Epiject by Abbott Laboratories.
- Syrup - Depakene by Abbott Laboratories its generic formulations include Apo-Valproic and ratio-Valproic.
Japan
- Tablets - Depakene by Kyowa Hakko Kirin
- Extended release tablets - Depakene-R by Kyowa Hakko Kogyo and Selenica-R by Kowa
- Syrup - Depakene by Kyowa Hakko Kogyo
Europe
In much of Europe, Dépakine and Depakine Chrono (tablets) are equivalent to Epilim and Epilim Chrono above.
Taiwan
- Tablets (white round tablet) - Depakine (Chinese: ???; pinyin: di-ba-dian) by Sanofi Winthrop Industrie (France)
Israel
Depalept and Depalept Chrono (extended release tablets) are equivalent to Epilim and Epilim Chrono above. Manufactured and distributed by Sanofi-Aventis.
India, Russia and CIS countries
- Valprol CR by Intas Pharmaceutical (India)
- Encorate Chrono by Sun Pharmaceutical (India)
- Serven Chrono by Leeven APL Biotech (India)
Brand names of valproate semisodium
- Brazil - Depakote by Abbott Laboratories and Torval CR by Torrent do Brasil
- Canada - Epival by Abbott Laboratories
- Mexico - Epival and Epival ER (extended release) by Abbott Laboratories
- United Kingdom - Depakote (for psychiatric conditions) and Epilim (for epilepsy) by Sanofi-Aventis and generics
- United States - Depakote and Depakote ER (extended release) by Abbott Laboratories and generics
- India - Valance and Valance OD by Abbott Healthcare Pvt Ltd, Divalid ER by Linux laboratories Pvt Ltd, Valex ER by Sigmund Promedica, Dicorate by Sun Pharma
- Germany - Ergenyl Chrono by Sanofi-Aventis and generics
- Chile - Valcote and Valcote ER by Abbott Laboratories
- France and other European countries -- Depakote
- Peru - Divalprax by AC Farma Laboratories
- China - Diprate OD

Research
As of 2016 it is also registered for 45 phase II clinical trials (some completed) for various cancers.
Source of the article : Wikipedia

EmoticonEmoticon